Data for A wrench in the works of human acetylcholinesterase: soman induced conformational changes revealed by molecular dynamics simulations
Electrostatic-field-embedded QM/MM calculations for the phosphonylation of human acetylcholinesterase by the nerve agent soman (GD)
In this illustration, the soman phosphorous and fluorine atoms are colored purple, oxygen atoms are red. In the reaction Histidine 447 takes the proton from Serine 203. The Serine 203 oxygen atom then interacts with the phosphorous atom (upper purple atom) of the soman. Then the fluoride ion of the soman (lower purple atom) leaves the phosphorous and interacts with a water molecule. The water molecule also forms a hydrogen bond (not shown) with the hydroxyl group of Tyrosine 124. Illustration: Jean-Luc Fattebert and Ed Lau.
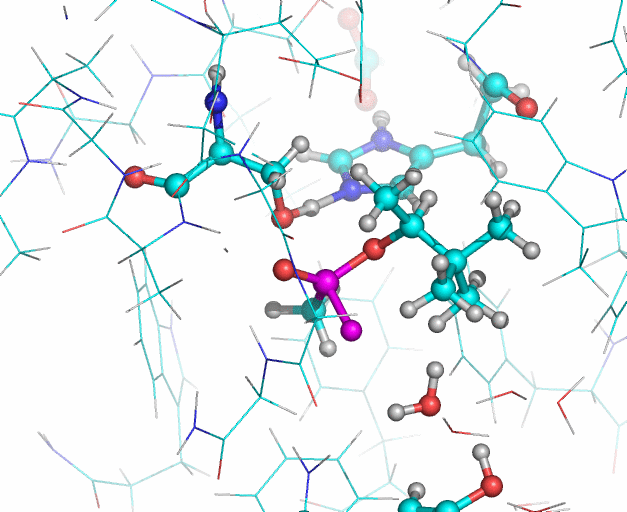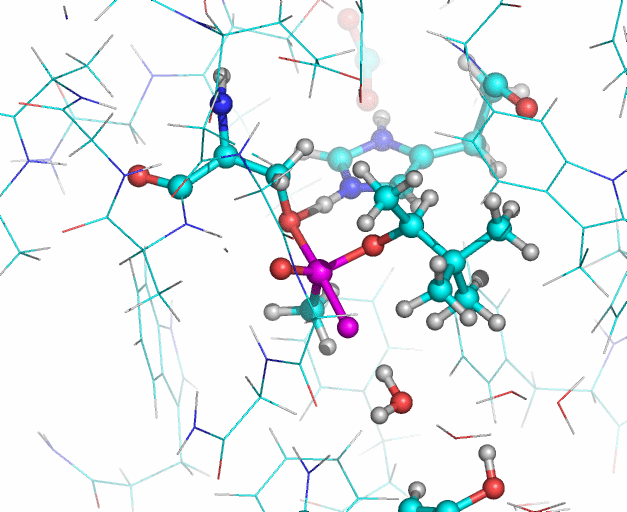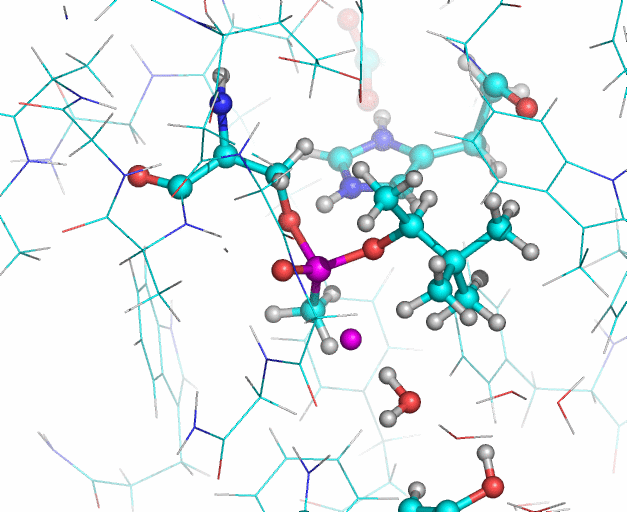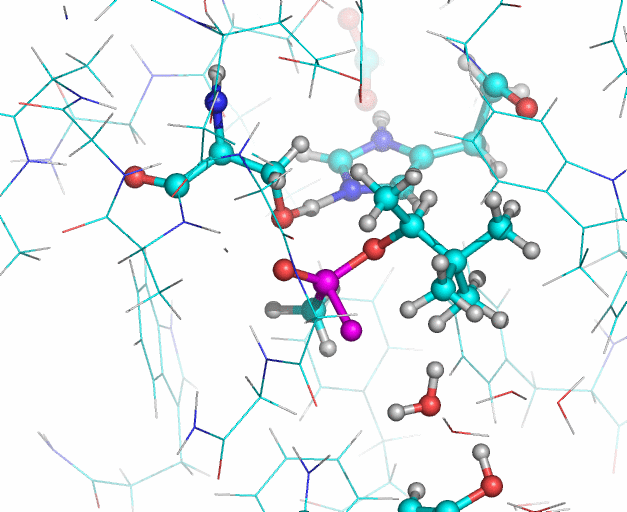
Equilibrated starting structures of apo human AChE and soman-adducted human AChE
NAMD topology files ("Protein Structure File")
Structures (Protein Data Bank format)
Source data - Figures 5 and 6
Xmgrace session files
allOmegaLoopCarmsdCombined.agr
mouthAreaApoSomanCombined.agr
BackDoorAreaCaAtomsCombined.agr
BackDoorAreaCombinedSideChains.agr
SideDoorAreaApoSomanCombined.agr
LLNL-WEB-667081


